History
The GHSG was founded in 1978 on the initiative of the German Federal Ministry of Education and Research (BMFT) with the aim to improve and standardize Hodgkin lymphoma diagnostics, treatment and follow-up care. The group was initially based in Hanover and started to conduct its first clinical multicenter trials on Hodgkin lymphoma under the direction of chairman Prof. Dr. Volker Diehl. While in the beginning only a small number of patients could be recruited for the trials, today a total of 40-60% of patients in Germany with newly diagnosed Hodgkin lymphoma are treated within the framework of the GHSG’s clinical trials.
When at the end of the 1970s scientists succeeded to cultivate malignant Hodgkin and Reed-Sternberg Zellen in vitro, this provided the first basis for discovering the exact pathobiological background of Hodgkin lymphoma. From then on, advancements in fundamental research and in Hodgkin lymphoma therapy were achieved hand in hand.
In 1983 the Trial Coordination Center moved to Cologne,
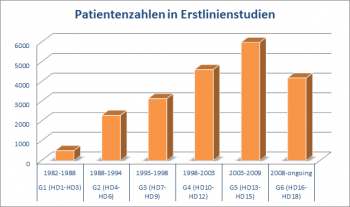
where the efforts to recruit as many patients as possible for its trials were stepped up. The study group was increasingly successful and gained a very good reputation. As a result, more and more patients could be included in the trials. While in the early 80s, only about 100 patients were recruited per year, in 2002 this number had increased to over 1000 patients from all over Germany and other European countries. The trial patients are recruited and enrolled by over 400 different participating treatment centers including, beside university hospitals, peripheral hospitals and oncology practices.
In 2007 Prof. Dr. Andreas Engert took over as chairman of the GHSG. Since then, the study group has been growing all the time, and its team now includes more than 30 employees.
It has continued its successful work, with numerous new clinical trials completed and published in renowned journals. Some milestones on its path were the introduction of new treatment standards for early stages (HD10), intermediate stages (HD14) and advanced stages (HD15) based on the trial results of the GHSG. Treatment optimization is a major goal, but apart from this the GHSG is now also increasingly focusing on late effects of treatment and the related scientific aspects. As a result of the continuous improvement in Hodgkin lymphoma therapy, there is meanwhile a very large number of long time survivors of the disease. Other key aspects of the GHSG’s current work are to investigate new biological and targeted agents and to further optimize radiotherapy and reduce radiation exposure if possible.